electron affinity exceptions|exceptions to orbital filling rules : iloilo Electron affinity measures the ease of gaining an electron by an atom. For example, the electron affinity of chlorine is −348.6 kJ/mol. The negative sign indicates that it is an .
KENO BONUS gives you a chance to increase your KENO winnings by 3, 4, 5 or 10 times. . Get a list of results for all of today’s drawings, or any day’s drawings. View Now. Winners By Day. Select the date and number of spots to see the winner counts for each prize. Sat, Aug 31, 2024 .
PH0 · trend for electron affinity
PH1 · periodic trends exceptions
PH2 · how to tell electron affinity
PH3 · exceptions to orbital filling rules
PH4 · exceptions to electron affinity trend
PH5 · electron affinity vs electronegativity
PH6 · electron affinity values periodic table
PH7 · Iba pa
For the 40th day prayer for the dead, the Philippines offers a variety of options. The first is a traditional Catholic prayer that can be performed alone or in groups. The second is the traditional Filipino folk prayer. The third is an alternative prayer that uses only one word: “God.” The first option is traditional. Read More »40th Day .
electron affinity exceptions*******Exceptions abound in electron affinity. Another case is in that of $\ce{F}$ versus that of $\ce{Cl}$. You would think that $\ce{F}$ being far more electronegative, would have the more negative electron affinity, but actually, that is not the case.
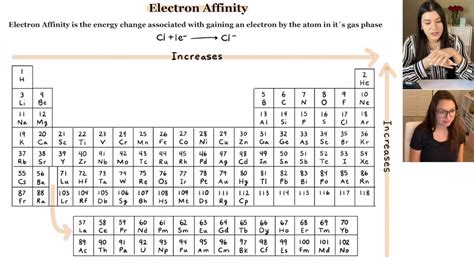
The electron affinity (EA) of an element is the energy change that occurs when an electron is added to a gaseous atom to give an anion. In general, elements with the . Electron affinity is a measure of the energy released when an extra electron is added to an atom. Electron affinities are measured in the gaseous state. In general, . Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative .The electron affinity (EA) is the energy change for adding an electron to a gaseous atom to form an anion (negative ion). This process can be either endothermic or exothermic, .electron affinity exceptions exceptions to orbital filling rulesElectron affinity measures the ease of gaining an electron by an atom. For example, the electron affinity of chlorine is −348.6 kJ/mol. The negative sign indicates that it is an . Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻( g ), the . The electron affinity trend is explained in this video with all the exceptions you need to know, the definition of electron affinity, how to write the chemic. Unlike some of these other properties, there are many exceptions to the trends for electron affinity. Electron affinity general increases moving across a row or period of the periodic table , until .

Exceptions to electron affinity trends include the noble gases, fluorine and Groups 2, 14 and 15 in the periodic table. Learning Outcomes. After completing this lesson, students.
The electron affinity trend is explained in this video with all the exceptions you need to know, the definition of electron affinity, how to write the chemic.
The chemical equation for electron affinity is as follows [1-4]. X (g) + e – → X – (g) When an electron approaches a neutral atom, the positively charged nucleus attracts it. The atom transforms into a negatively .Electron affinity is the energy change that occurs when an electron is added to a gaseous atom. For many atoms, electron affinity is exothermic. . Periodic Trends of Electron Affinity . There are many .The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron addition process is. Not all elements form stable negative ions in which case the electron affinity is zero or even positive. The three factors affecting the electron affinity of a molecule are Nuclear Charge, Atomic Size, and Electronic Configuration. Nuclear Charge: The greater the nuclear charge, the greater will be the attraction of the incoming electron. This will result in a larger value of electron affinity. To explain it in simpler terms, the nuclear charge .
The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom: E(g) +e− → E−(g) energy change=EA (1.1.2.4.1) (1.1.2.4.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .The electron affinity (EA) is the energy change for adding an electron to a gaseous atom to form an anion (negative ion). . EAs tend to become more negative. The exceptions found among the elements of group 2 (2A), group 15 (5A), and group 18 (8A) can be understood based on the electronic structure of these groups. The noble gases, .electron affinity exceptions Exceptions to electron affinity trends include the noble gases, fluorine and Groups 2, 14 and 15 in the periodic table. Learning Outcomes. After completing this lesson, students should be able to:
Convierte 150 USD a MXN con el conversor de moneda de Wise. Analiza la evolución y el estado actual del tipo de cambio de dólares estadounidenses/pesos mexicanos y recibe, sin coste, alertas por correo electrónico sobre el estado del tipo de cambio.⭐ 20.000+ Lei în oferte pariuri sportive ⭐ Top 43 case de pariuri sportive online ⚽ Pariuri sportive fotbal ⭐ Cum se joacă la pariuri?
electron affinity exceptions|exceptions to orbital filling rules